Therefore a carbon ion picks up 4 electrons to become C-4. How many electrons does carbon lack in its outer shell.
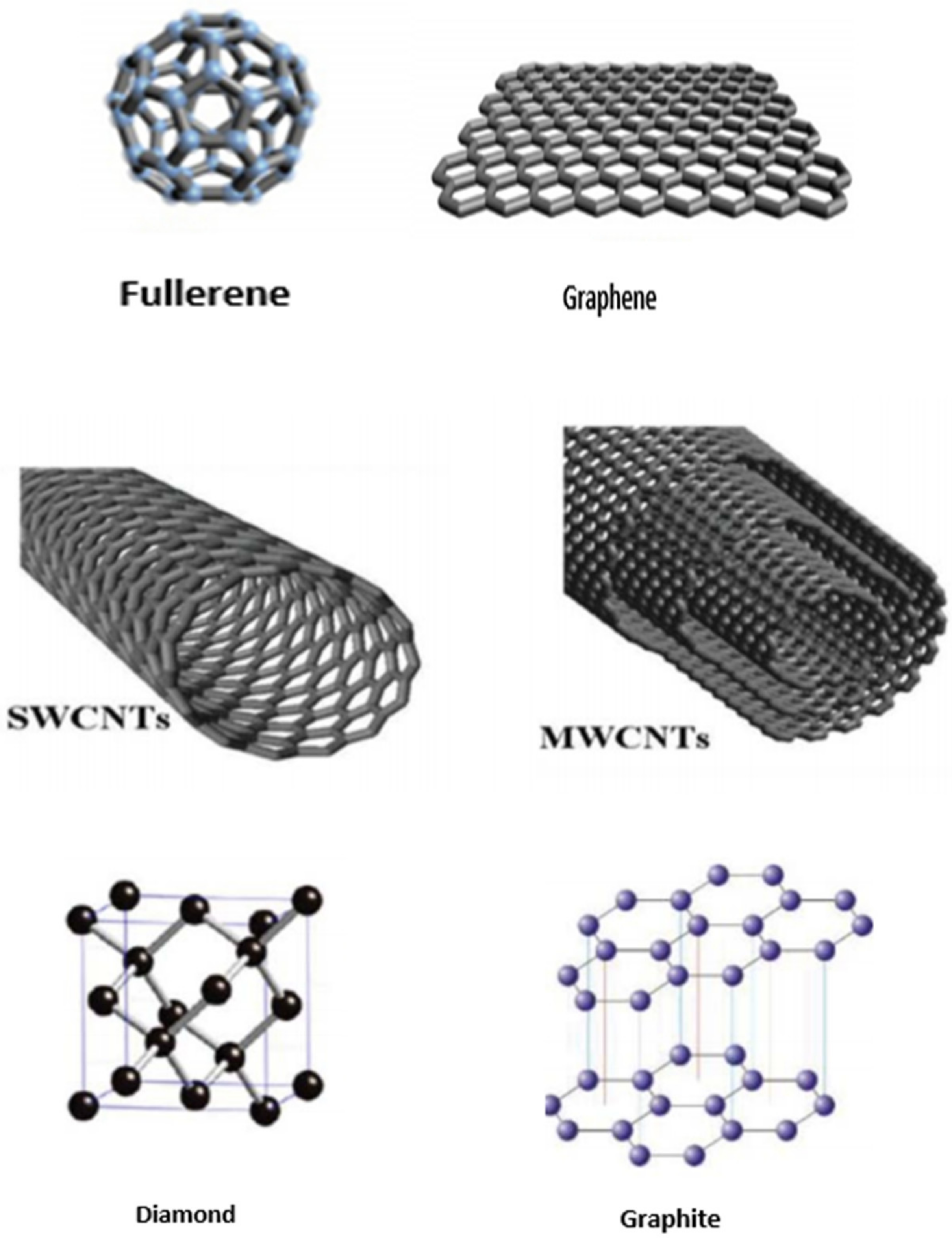
Nanomanufacturing Free Full Text Synthetic Approach To Rice Waste Derived Carbon Based Nanomaterials And Their Applications Html
Number of electrons in the innermost shell.

. Carbon lack 4 electrons in its outer shell. 4154017 Views. Thus the number of electrons lacked by carbon atom 8-4.
Neutral carbon has no electrons. How many electrons does carbon lack in its outer shell. 1 question How many electrons does carbon lack in its outer shell.
See also why are the states shaped the way they are. However the correct answer is 4. Since Carbon only has 4 of its outer electron slots or valence electrons full it has room to make bonds with 4 other atoms assuming they are all single bonds.
An atom of carbon can share four electrons. How many innercore and outervalence electrons are in fe. Carbon does not have five valence electrons.
Updated 932020 33600 PM. A valence electron is always unpaired until it forms a bond and every unpaired electron must be in a separate orbital. According to the periodic table Carbon has an electron configuration of 1s2 2s2 2p2 however it forms a.
A neutron carbon doesnt lack any electrons. To see more answers head over to College Study Guides. The atomic number of Carbon C is 6.
The outer shell or the valence shell contains 4 electrons. Magnesium is in period three so we will only consider shells up to. It has the same number of electrons as neutrons.
The theory stated that combustable objects contained a material called phlogiston a substance without mass color odor etc. In that sense it needs four electrons to complete the second shell. Carbon as a non-metal wants to gain electrons to have 8 outer shell electrons.
It has exactly the same number of electrons as it has neutrons. Weathering and erosion d. How many electrons does carbon lack in its outer shell.
Therefore the shell energy diagram will look like the following. When atoms form ions they lose or gain electrons. Up to 256 cash back how about transition metal ions.
The phlogiston theory in the 17th century attempted to explain burning. The characteristic way in which atoms of an element react is most related to the a. Which turns sediments into sedimentary rock.
The phlogiston theory was accepted until the 18th century when lavoisier. In its outer shell carbon has 4 electrons. Pressure and heat c.
This will fill Carbons valence shell and give it. The octet rule states that atoms can fill their outer shells with up to 8 electrons a full shell of 8 is the most stable configuration. Size of the nucleus.
A neutral carbon doesnt lack any electrons. 2 in the first and remaining 4 in the outer shell. The highest filled energy shell will be the outermost electron shell and we then count the number of electrons in that shell.
A calcium atom has 20 protons and 20 electrons. The electronic configuration depicts that k shell of the carbon atom contains 2 electrons. Asked 932020 31128 PM.
A To solve this problem we must put the number of electrons a magnesium atom has into the appropriate shells. The atomic number of carbon is 6. Here is your answer.
Number of electrons in the outermost shell. Every atom wants to possess 8 electrons in its valence shell in order to become stable. 32 Votes Answer and Explanation.
In that sense it needs four electrons to complete its second shell. Carbon has four electrons in its outer shell. After objects burned the objects were dephlogistonated and were then in their true form.
So electronic configuration of Carbon is 2 4. However it has four electrons in its outer shell in comparison with eight electrons for a noble gas. Ca2 represents an ion with 20 protons and 18 electrons.
VandarPoints 911 Log in for more information. Log in for more information. It can be concluded that carbon lacks 4 electrons in its outer shell.
Carbon can only form four valence orbitals and when these pair it forms four bonds and has access to eight electrons. How many electrons does carbon lack in its outer shell. Carbon has two electrons in its inner shell and four electrons in its outer shell.
Carbon has a total of six electrons with two in the first orbital and four in the second orbital. So carbon lacks 4 electrons in its outermost shell. However four electrons are an outer shell compared to eight electrons for a noble gas.
Number of neutrons in the nucleus. It has the same number of electrons and neutrons. How many electrons does carbon lack.
THIS IS THE BEST ANSWER. How Many Electrons Does Helium Have In Its Outer Shell. Select all that apply Choose from the following list the most common elements which bond to carbon.
The 2 charge next to the symbol indicates a loss of two electrons. Compaction and cementation b. How many electrons does carbon lack in its outer shell.
Asked 11112014 104857 AM.
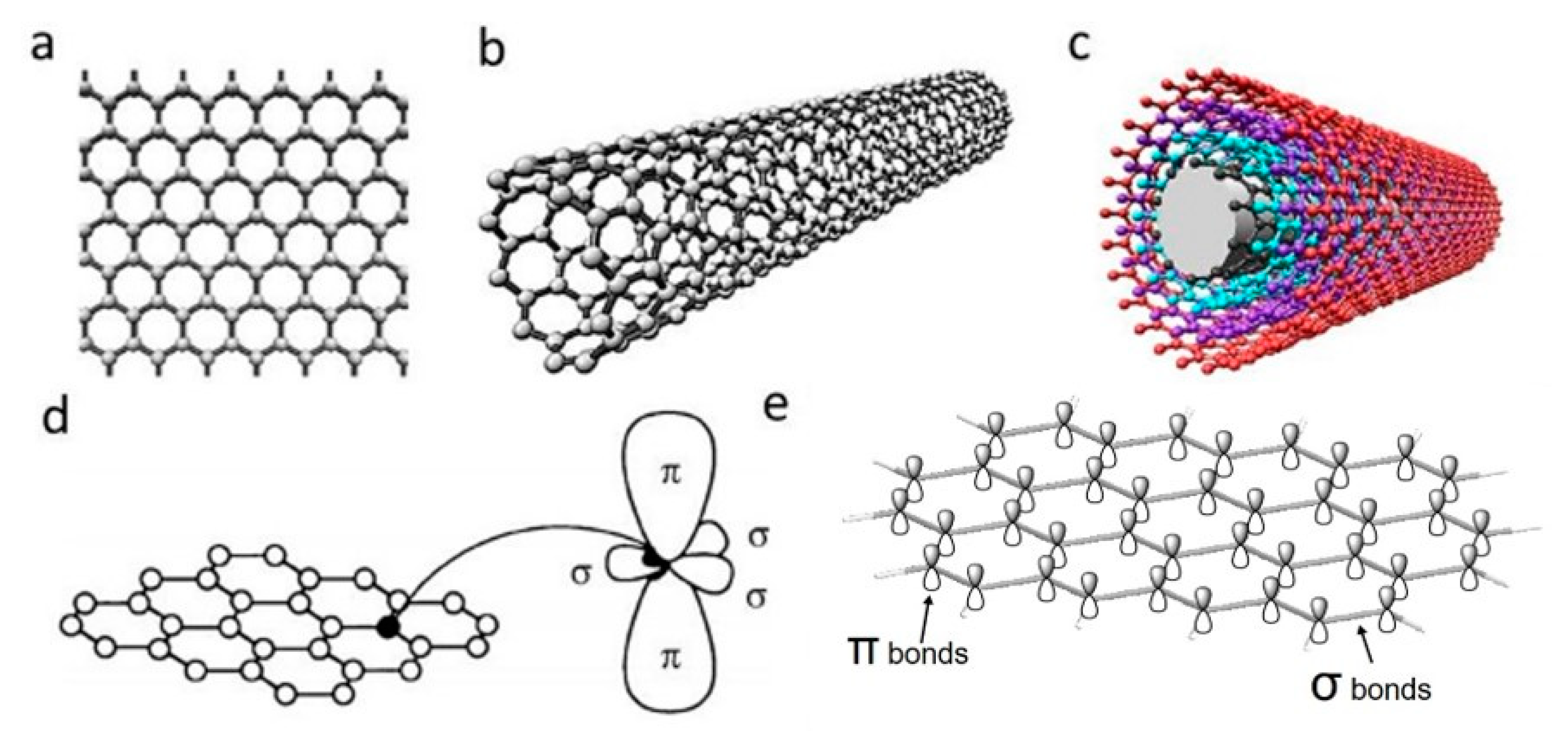
Polymers Free Full Text Carbon Nanomaterials For Electro Active Structures A Review Html

Key Factors And Primary Modification Methods Of Activated Carbon And Their Application In Adsorption Of Carbon Based Gases A Review Sciencedirect

Polubienia 3 555 Komentarze 19 정재현 130notes Na Instagramie I M So Unproductive Study Notes Study Inspiration Study Space
0 Comments